Addressing Diversity in Clinical Research: Data for Alignment with New FDA Guidelines

At PolicyMap, we are committed to supporting BioPharma companies and Clinical Research Organizations (CROs) in meeting new diversity requirements in clinical trials. With the recent FDA draft guidance, “Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies Guidance for Industry,” ensuring studies are inclusive and representative of all populations is more crucial than ever. This policy update, coupled with increasing awareness of disparities in clinical research access, underscores the urgent need for a more equitable distribution of research opportunities. Recently, Clinical Trials Arena highlighted the unequal access to clinical trials across the United States, underscoring the urgent need for more equitable distribution of research opportunities.
Adding to these challenges is the reality of healthcare deserts in the U.S., where access to basic healthcare services is severely limited. Healthcare deserts, often found in rural areas and underserved urban neighborhoods, contribute to unequal access to clinical trials. According to an analysis from GoodRx Research, more than 80% of counties across the U.S. lack proper access to the services needed to maintain health.
So, how can PolicyMap help? Our robust data capabilities continue to be a vital resource for:
Identifying and Understanding Diverse Populations
Understanding the demographic landscape is the first step in creating more inclusive clinical trials. PolicyMap provides comprehensive data on various population characteristics, including age, race, ethnicity, and health risks and outcomes. This data helps BioPharma companies and CROs pinpoint underrepresented communities and tailor their outreach efforts accordingly.
Enhancing Community Engagement Strategies
Building trust and engagement within diverse communities is crucial for successful recruitment and retention in clinical trials. PolicyMap’s data can inform more effective community engagement strategies by highlighting areas with high potential for participation. Quickly identify community resources, health services, local organizations, and nonprofits, and shift your focus to establishing meaningful connections and fostering collaboration.
Optimizing Recruitment Efforts for Broad Representation
Recruitment is often one of the biggest challenges in clinical research, especially when aiming for diversity. PolicyMap’s data-driven insights enable researchers to optimize their recruitment tactics. By understanding where diverse populations live and what barriers they might face, such as limited access to healthcare facilities or transportation issues, researchers target campaigns for the best chance of success.
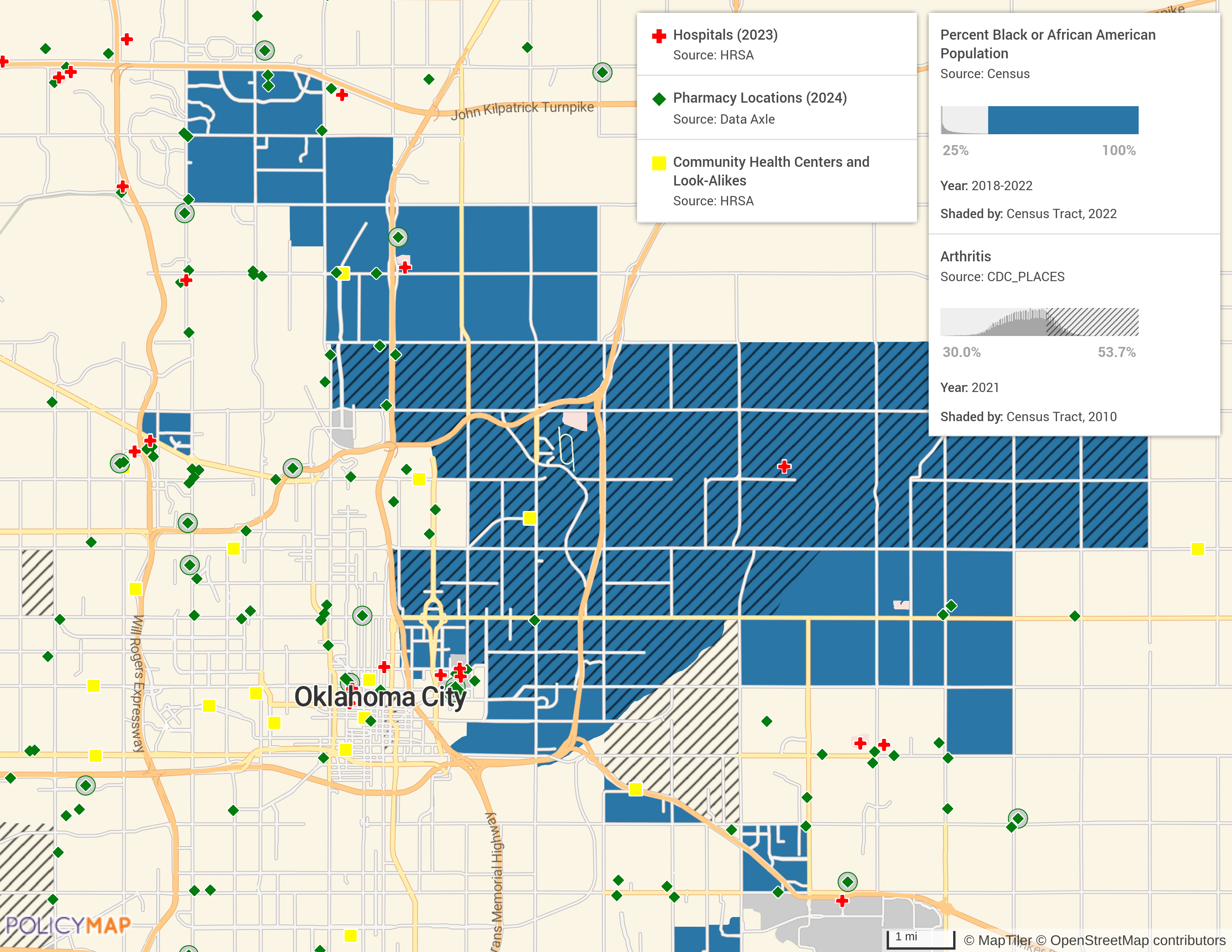
Documenting Evidence of Due Diligence
PolicyMap offers comprehensive data visualization and mapping tools that can assist your organization in demonstrating the rationale behind specific trial site selections. By incorporating PolicyMap into your clinical trial planning, you can enhance compliance with FDA guidelines by providing clear evidence of efforts to reach diverse populations. Find sites for clinical trial sites located in areas with higher concentrations of underrepresented racial and ethnic patients and indigenous populations. Generate detailed reports and visual maps to showcase your strategic site selection process and commitment to diversity.

Locating Mobile Clinical Trial Sites
PolicyMap’s robust data capabilities make it an invaluable tool for planning mobile clinical trial sites. By analyzing demographic, socioeconomic, and healthcare infrastructure indicators, organizations can strategically place mobile units to maximize accessibility and ensure they are positioned near essential services and transport routes. This approach helps in overcoming logistical barriers, enhancing participant recruitment, and ensuring compliance with FDA diversity guidelines.
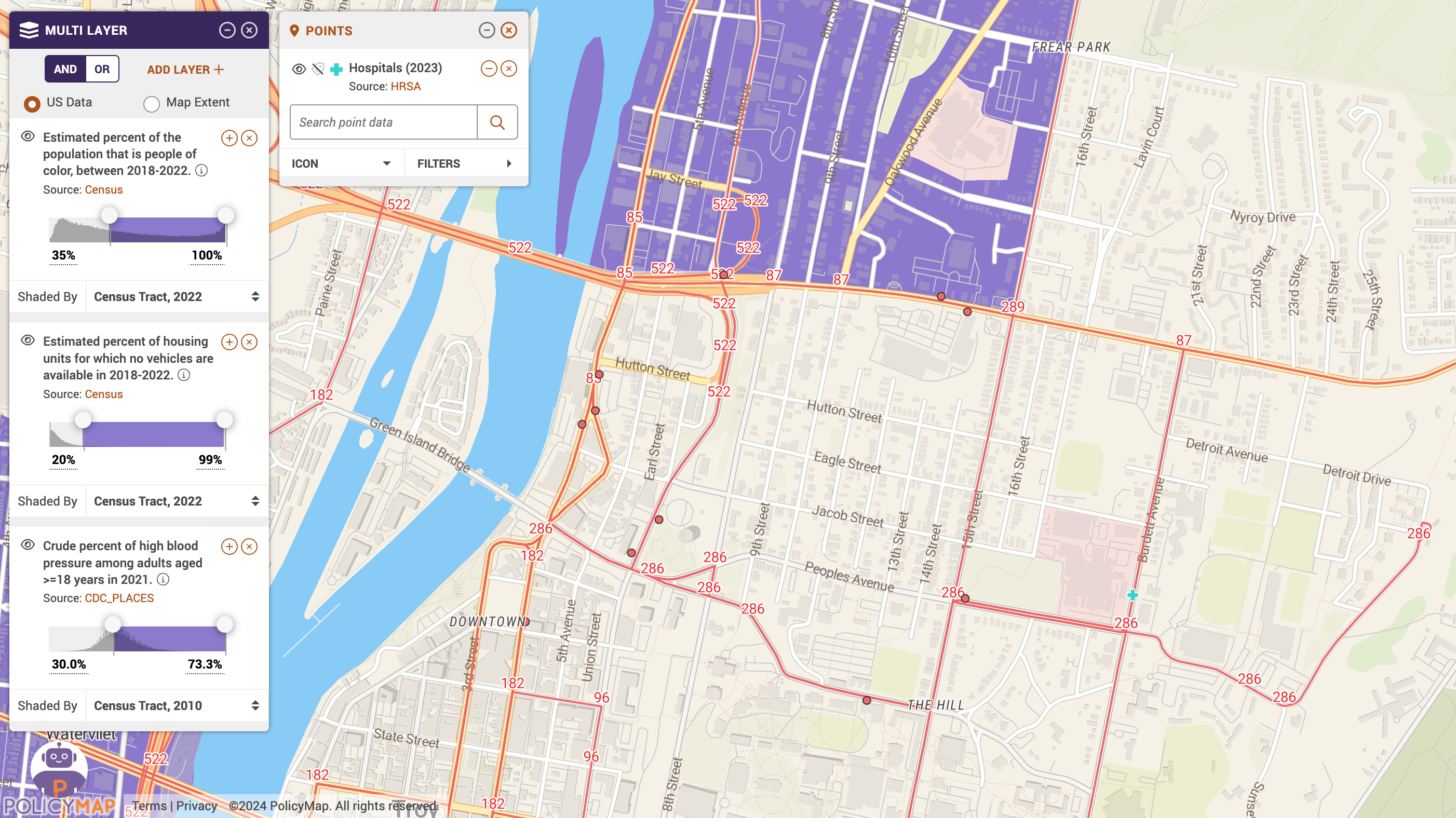
Supporting the Move Toward Greater Inclusivity
Let’s work together to bridge the gap and create more inclusive and effective clinical trials!
We’re thrilled to see the industry moving toward greater inclusivity, and we’re here to help you navigate these changes effectively. Our solution can help you meet regulatory requirements but also contribute to the overall goal of improving healthcare outcomes for all populations. Pave the way for more equitable healthcare advancements with clinical trials that are compliant and genuinely inclusive.
Contact Us
In a landscape where the benefits of clinical research should be accessible to everyone, PolicyMap stands out as a key partner in making this vision a reality. Fill in the form below to schedule your one-on-one tutorial and learn how PolicyMap can help you achieve your diversity action plan goals.